Your LIMS Upgrade Questions,
Answered
White Paper Request
Fill The Form Below To Receive The White Paper
What It's About
When you’re considering upgrading your LIMS, the decision-making process may involve a lot of questions. This white paper provides answers to 19 of the most common upgrade questions, including the following:
- How do I know if it’s time to upgrade my LIMS?
- What wording should be included in the vendor contract to ensure costs are controlled during the upgrade?
- What should be done with the legacy information?
- And 16 more answers!
Who it’s for
Request this white paper to ensure your LIMS upgrade goes as smoothly as possible. There will always be some hiccups and delays, but forewarned is forearmed.
How to use it
This white paper illustrates some of the common techniques for data visualization in biotechnology and pharmaceutical laboratory settings. It also provides examples of the types of data that can be conveyed with visualizations.
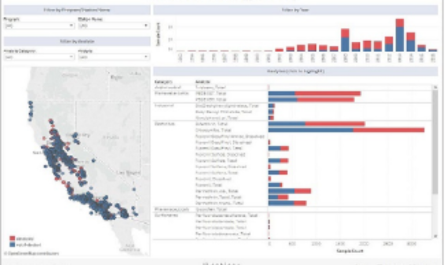
Frequently Asked Questions
Is this really free?
Absolutely! We're sharing this free knowledge that we hope you'll find useful. Keep us in mind next time you have LIMS questions.
Why do I need to share my information?
We ask for your personal information so we can provide you with important details about the white paper, including access links and any follow-up materials. Your information also helps us tailor future content to your needs. Rest assured, your privacy is important to us, and we will never share your information without your consent.
Who wrote this white paper?
Our white papers are always written in-house with the assistance of our expert consultants.
.png?width=850&height=124&name=cropped-CSolsLongLogo_rgb_taglinesecondary_tagline3-2-resize%20(1).png)
